Abstract
Constitutional ring chromosomes originate from any chromosome and are formed by breakage and fusion of both chromosome arms, usually with associated loss of chromosomal material at the telomeres. They occur in approximately 1:50,000 foetuses, but their true incidence in live births is largely unknown, as not all carriers display an abnormal phenotype. Constitutional ring chromosome 21, r(21)c, occurs in de novo and inherited forms. A wide range of phenotypic features have been described in association with r(21)c, including developmental delays, learning difficulties and a range of medical conditions. However, data on the association of constitutional ring chromosomes and cancer are lacking. A literature search of 67,323 abnormal cancer karyotypes revealed five cases of r(21)c with ALL (n=3) and acute myeloid leukaemia (n=2). In addition, we previously identified three r(21)c carriers among 530 patients with iAMP21-ALL in an international study. The iAMP21-ALL subtype accounts for 2% of childhood precursor-B ALL, therefore these three cases represent around 0.5% of iAMP21-ALL. As r(21)c have not been reported in other ALL subtypes, their incidence in ALL overall is extremely low. However, this strong association with iAMP21-ALL warrants further study.
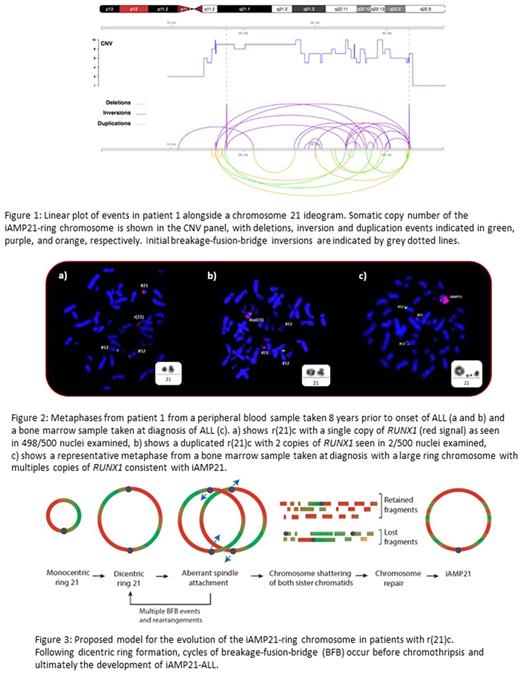
From an international search for r(21)c carriers with acute leukaemia, we identified four cases with ALL, including the three from the international study. All were classified as iAMP21-ALL according to the standard definition. Their median age was 8 years (range 5-10 years) at diagnosis and their white blood cell counts (WCC) were low. These features, of older age and low WCC, are typical of patients with iAMP21-ALL. Abnormal acquired karyotype information was only available for two patients. Both were complex and of note was that the r(21)c was extensively rearranged into a grossly abnormal chromosome 21, as also seen in the karyotypes of previously published cases, as demonstrated by karyotype, FISH and SNP6.0 arrays. SNP6.0 data from the diagnostic samples of the same two patients confirmed the characteristic profile of iAMP21, which we have previously reported, including deletion of the telomeric region of chromosome 21, associated with the copy number profile of gains and losses along chromosome 21, typical of chromothripsis. True evidence of the characteristic mechanism of iAMP21 chromosome formation, resulting from telomeric loss, the presence of a fold back loop at the telomeric breakpoint indicative of a breakage-fusion-bridge cycle, and all types of rearrangements typical of chromothripsis, were confirmed from whole genome sequencing (WGS) (Figure 1). SNP6.0 arrays and WGS of the germline samples indicated constitutional telomeric loss as a consequence of ring chromosome formation. This same breakpoint, conserved in the leukaemic sample, provided the initiating step of the iAMP21-ring chromosome. A similar telomeric deletion was observed in the germline sample of a third carrier, although no diagnostic DNA was available from the leukaemic sample.
Patient 1 showed evidence that cross overs, in the form of sister chromatid exchanges during DNA replication, occurred between chromatids, which led to the formation of a dicentric chromosome in the germline sample. This was confirmed by cytogenetics and FISH analysis of a peripheral blood sample from patient 1, taken 8 years before the onset of ALL, where 2/500 nuclei showed evidence of a duplicated ring (Figure 2). The formation of a dicentric chromosome is an early step in the development of iAMP21 chromosomes. As the two centromeres move to opposite poles during anaphase, an anaphase bridge is produced and, depending on where breakage and reunion occurs, duplicated segments may form, followed by chromothripsis (Figure 3), as we observed in the diagnostic sample of this patient and as also seen in cases of sporadic-iAMP21. In the same way as we described previously for carriers of the constitutional Robertsonian translocation, rob(15;21)c, it appears that r(21)c chromosomes are also predisposed to iAMP21-ALL, through their propensity to undergo chromothripsis after replication. The mode of initiation of these processes needs to be unravelled in order to fully understand these intriguing predispositions to iAMP21-ALL, which may provide insight into how other complex rearrangements arise in cancer.
Hunger: Erytech Pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Honoraria; Novartis: Consultancy; Amgen: Consultancy, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal